Antibody
Antibody
Il primo ciclo di MAbs biosimilari e proteine di fusione con date di scadenza del Brevetto tra il 2012-2018 include RITUXIMAB, TRASTUZUMAB, ETANERCEPT, INFLIXIMAB, BEVACIZUMAB, CETUXIMAB e ADALIMUMAB.
Antibodies for type 2 diabetes
About type 2 diabetes
The condition is a chronic endocrine disorder characterized by high blood sugar (hyper-glycemia) due to diminished effects of insulin, the hormone responsible for regulating sugar levels in the blood. Type 2 diabetes is considered insulin-independent, as the issue is cell desensitization to the hormone, which means increased levels of the hormone do not improve the condition. Around 380 million people globally are estimated to have type 2 diabetes, which is the majority of people affected by diabetes. According to an estimation from WHO, more than 1.5 million people die annually as a direct consequence of diabetes.1
Market potential
The American Diabetes Association recently published a report in which the total cost of patients diagnosed with diabetes in 2017 was estimated at USD 327 billion. This included USD 237 billion in direct medical costs and USD 90 billion in reduced productivity.2 While risk factors for type 2 diabetes include genetic predisposition and obesity, nearly 90% of patients are treated with pharmacological means rather than lifestyle adjustments alone.3 4
Therefore, the large therapeutic market for type 2 diabetes is thought to amount to over USD 26 billion annually in the EU5 (France, Germany, Italy, Spain and UK), Japan and the US combined.
1. World Health Organization. Global report on diabetes. World Health Organization, 2016.
2. American Diabetes Association. “Economic Costs of Diabetes in the US in 2017.” Diabetes care 41.5 (2018): 917.
3. Datamonitor Healthcare’s proprietary diabetes survey, May 2016.
4. Datamonitor Healthcare’s Forecast: Type 2 Diabetes 2016–25
Antibody Mediated Oral Delivery of Therapeutic DNA for Type 2 Diabetes Mellitus
Type 2 diabetes mellitus (T2DM) is a chronic and progressive hyperglycemic condition. Glucagon-like peptide-1 (GLP1) is an incretin secreted from pancreatic β-cells and helps to produce insulin to balance the blood glucose level without the risk of hypoglycemia.
However, the therapeutic application of GLP1 is limited by its intrinsic short half-life and rapid metabolic clearance in the body.
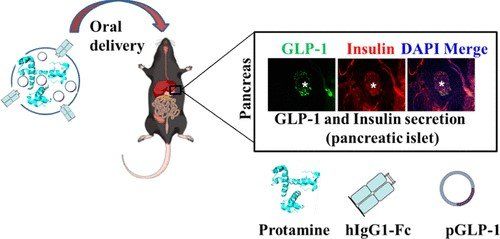
To enhance the antidiabetic effect of GLP1, we designed a human cysteine-modified IgG1-Fc antibody-mediated oral gene delivery vehicle, which helps to produce GLP1 sustainably in the target site with the help of increased half-life of the Fc-conjugated nanocarrier, protects GLP1 from acidic and enzymatic degradation in the gastrointestinal (GI) tract, uptakes and transports the GLP1 formulation through the neonatal Fc receptor (FcRn), and helps to release the GLP1 gene in the intestine.
Our formulation could reduce the blood glucose from about an average of 320 mg/dL (hyperglycemic) to 150 mg/dL (normal blood glucose concentration) in diabetic mice, which is about 50% reduction of the total blood glucose concentration.
GLP1 (500 μg) complexed with the IgG1-Fc carrier was proven to be the optimal dose for a complete reduction of hyperglycemic conditions in diabetic mice. A significant amount of insulin production and the presence of GLP1 peptide were observed in the pancreatic islets of oral GLP1 formulation-treated diabetic mice in immunohistochemistry analysis compared to nontreated diabetic mice.
Delivery of DNA has emerged as a novel therapeutic for diverse applications. We designed a recombinant human IgG Fc fragment to attain neonatal FcRn receptor (FcRn) mediated active uptake and transport of plasmid DNA (pDNA).
we developed an oral GLP-1 gene delivery system on the platform of cationic hIgG1-Fc-9Arg. Prolonged t1/2, less immunoactivity, and better bioactivities of hIgG-Fc-9Arg/pGLP-1 complexes appeared to be a promising approach to achieve potent treatment of type 2 diabetes treatment.
Human monoclonal antibodies that bind allosterically to the insulin receptor (INSR): XMetA, XMetS and XMetD
Many therapeutic monoclonal antibodies act as antagonists to receptors by targeting and blocking the natural ligand binding site (orthosteric site). In contrast, the use of antibodies to target receptors at allosteric sites (distinct from the orthostericsite) has not been extensively studied.
XMetA directly activates the INSR either alone or in combination with insulin. XMetS, in contrast, does not directly activate the INSR but markedly enhances the receptor’s ability to bind insulin and potentiate insulin signaling. Both XMetA and XMetS are effective in controlling hyperglycemia in mouse models of diabetes.
A third allosteric antibody, XMetD, is an inhibitor of INSR signaling.
allosteric antibodies to INSR can modulate its signaling and correct conditions of glucose dysregulation.
There is possibility that the use of allosteric antibodies can be expanded to other receptors for the treatment of metabolic disorders.
Anti-ACVR2B ADCC Therapeutic Antibody (Bimagrumab BYM338)
This product is an ADCC enhanced antibody produced by our PLANT platform.
Recombinant human monoclonal antibody expressed in Plant binding to human ACVR2B. Bimagrumab is a human monoclonal antibody developed to treat pathological muscle loss and weakness.
It targets the activin type II receptor (ActRII)A and ActRIIB, preventing binding to their natural ligands which negatively regulate muscle growth, including myostatin, growth and development factor 11, and activin. It has been used in trials studying the treatment and supportive care of sarcopenia, skeletal muscle, mechanical ventilation, sporadic inclusion body myositis, and sporadic inclusion body myositis (sIBM), among others.
Anti-human SeP monoclonal antibody AE2
Anti-human SeP monoclonal antibody AE2 as with neutralizing activity against SeP
Selenoprotein P (SeP) functions as a selenium (Se)-supply protein.
SeP is identified as a hepatokine, promoting insulin resistance in type 2 diabetes. Thus, the suppression of Sesupply activity of SeP might improve glucose metabolism. Here, we develop an anti-humanSeP monoclonal antibody AE2 as with neutralizing activity against SeP. Administration of AE2to mice significantly improves glucose intolerance and insulin resistance that are induced by human SeP administration. Furthermore, excess SeP administration significantly decreases pancreas insulin levels and high glucose-induced insulin secretion, which are improved by
AE2 administration. Epitope mapping reveals that AE2 recognizes a region of human SeP adjacent to the first histidine-rich region (FHR). A polyclonal antibody against the mouse SeP FHR improves glucose intolerance and insulin secretion in a mouse model of diabetes. This report describes a novel molecular strategy for the development of type 2 diabetes therapeutics targeting SeP.
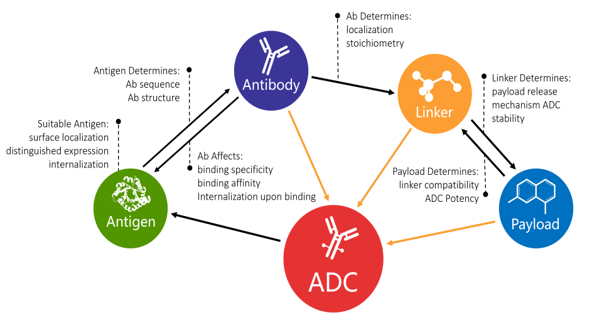
Antibody Drug Coniugate
ADC with cytotoxin
INTERLEUKINE (IL-11; IL 4 ;IL2)
Aequilibriu pharma VIRUS-LIKE PARTICLES
Virus-Like Particles (VLPs) Production in Plant System
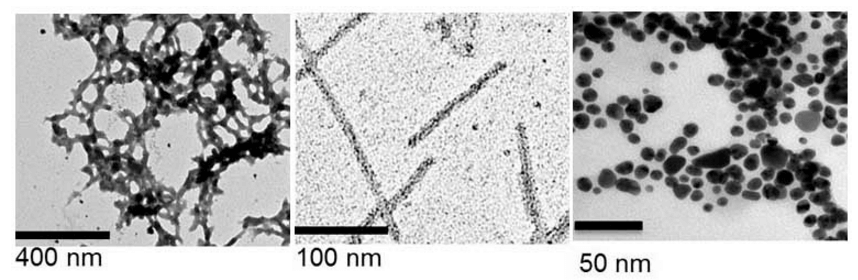
Virus-Like Particles (VLPs) for Vaccine Development
OUR SYNTHETIC VHH NANOBODY (The single-chain VHH Abs)
Aeqpharma select and validate of nanobodies derived from a fully synthetic, proprietary human VHH antibody library. The single-chain VHH Abs (nanobodies) can be easily fused to a tag (ie. fluorescent markers, enzymes, multi-Fc species) resulting in fully-functional recombinant nanobodies for a wide-range of in vitro and in vivo applications.
VHH NANOBODY PROPERTIES
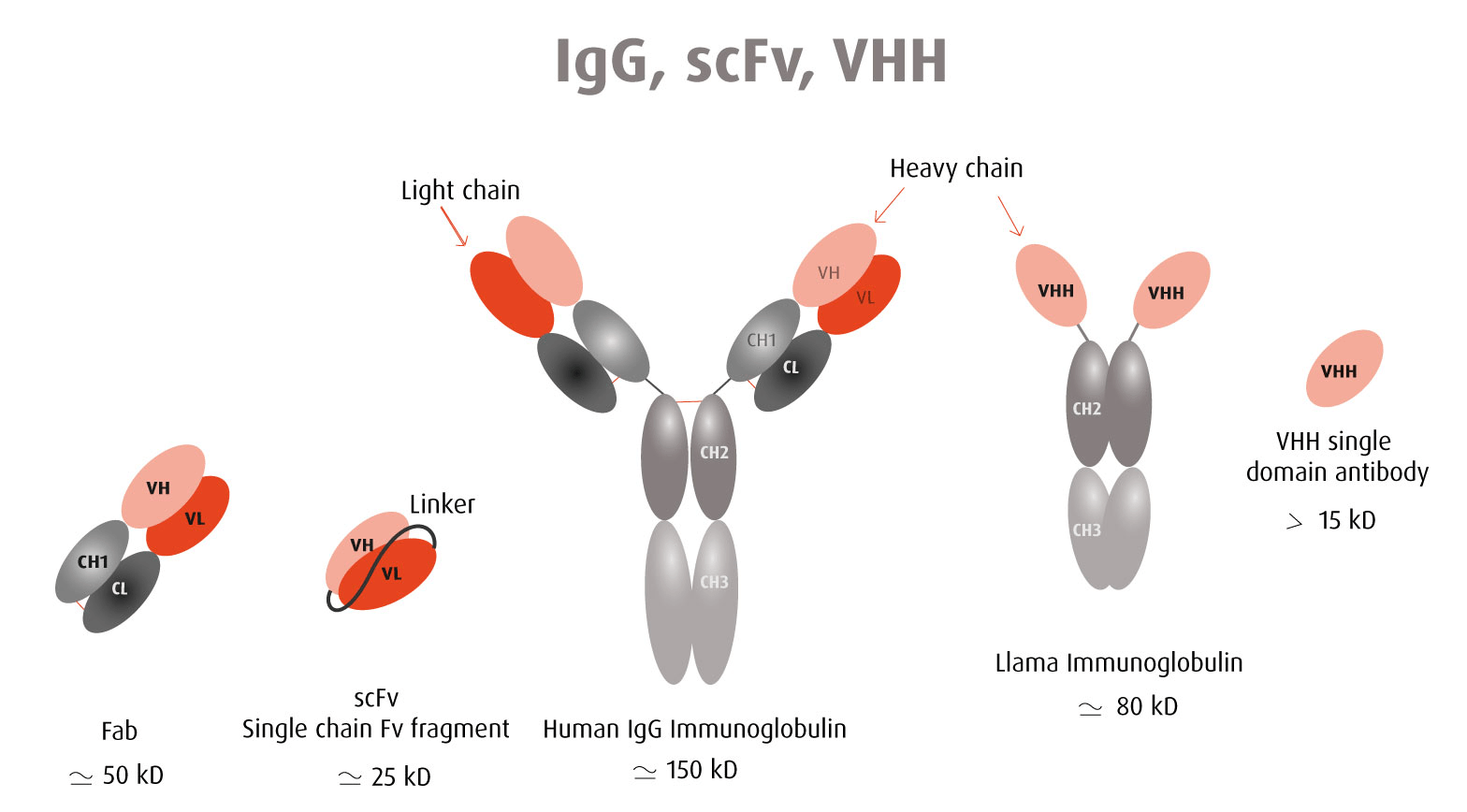
Monoclonal antibodies are large molecules of about 150 kDa and it sometimes limits their use in assays with several reagents competing for close epitopes recognition. Because of these limitations, the use of smaller antibody-derived molecules has emerged.
Comparison between a VHH antibody and a full immunoglobulin
Single-chain variable-fragment (scFv) antibodies have been commonly used as alternatives. ScFv consist of only the light chain and heavy chain variable regions of immunoglobulins connected by a peptide linker. Their average molecular weight is about 27 kDa. ScFv contain the antigen-binding site and are asspecific and affine as intact antibodies.
More recently, single domain antibodies were isolated from camelid animals; the so-called VHH. A VHH antibody corresponds to the variable region of a heavy chain of a camelid antibody and has a very small size of around 15 kDa - hence the name "nanobody".
The advantage of these antibody-derived molecules is their small size which enables their binding to hidden epitopes not accessible to whole antibodies. In the context of therapeutic applications, a small molecular weight also means an efficient penetration and fast clearance. A VHH nanobody has a higher probability of adopting identical intra- and extracellular folding. Therefore, it is a qualified candidate for intracellular probing (Intrabody).
Being a single-domain antibody molecule, a VHH nanobody is expressed in cell without the need for a supramolecular assembly in contrast to a full immunoglobulin made of 4 chains, 2 light chains and 2 heavy chains. A VHH nanobody is more stable and robust than a whole antibody.
Both scFv and VHH nanobodies can be linked to the Fc fragment of the desired species and keep their specificity and binding properties (Minibody).