Sense of the life
AEQUILIBRIUM PHARMABiosimilar Solution and Pioneering BiomoleculesAequilibrium pharma is a privately held biotech company, founded in 2018 in Milan,Italy. We are a drug discovery startup that focuses on the potential of monoclonal Antibody and Eexosomes as new treatment options for intractable diseases. Unlike private-originated ventures, we were founded with a market-in approach.We are committed to finding treatments for severe diseases with large unmet clinical needs such as pain and aggressive metastatic cancer using our unique angle of a targeted functionality antibody and Exosomes approach.
Products
BUSINESS MODEL
Our company is engaged in two business areas: the "Drug Discovery Business," which aims to commercialize exosomes as pharmaceuticals, and the "Non-Drug Discovery Business," which sells exosomes as raw materials for non-medical products.
The "Drug Discovery Business" consists of two business models: the "Asset Model," where we conduct clinical trials of our assets, and the "Platform Model," where we license our exosome technology.
Among these, the "Asset Model" is the driver of our medium- to long-term growth. Although research and development investments precede revenue, we aim to increase the value of our assets by obtaining evidence of safety and efficacy in clinical trials targeting diseases with high unmet medical needs. By licensing global rights to pharmaceutical companies, we expect to generate significant revenue.
On the other hand, while the future revenue scale of the "Platform Model" and "Non-Drug Discovery Business" is limited compared to the "Asset Model," they enable us to acquire stable cash flow early with less research and development investment, which is expected to contribute to the improvement of our financial health.
By adjusting the weight of these multiple business models according to our growth stage, we aim to achieve continuous growth over the medium to long term while controlling risks.

About Exosome
Introduction to Exosomes
Exosomes are a type of extracellular vesicle (EV) primarily utilized for intercellular communication. These vesicles are found in many eukaryotic cells and are involved in various physiological processes. This section introduces the characteristics and roles of exosomes.
Origin and Structure
Exosomes are membrane vesicles formed from a portion of intracellular vesicles called endosomes and released extracellularly. They are enclosed by a lipid bilayer containing various molecules derived from the cell. Their diameter ranges from 50 to 200 nanometers, about one-hundredth the size of a cell.
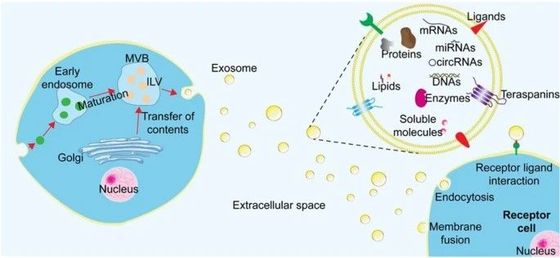
Composition
The lipid bilayer enclosing exosomes originates from the cell membrane of the secreting cell (hereafter referred to as the "secreting cell") and consists of components similar to the cell membrane. Various proteins exist on the surface of the lipid bilayer, some of which function as membrane surface markers. Notably, tetraspanins, four-transmembrane proteins, are abundant in exosomes. Tetraspanins include CD9, CD63, CD81, and others. The contents of exosomes are derived from the secreting cell, including nucleic acids (DNA, mRNA, microRNA), proteins, and more.

Function: Intercellular Communication
After secretion from the secreting cell, exosomes travel through body fluids and deliver their contents to another cell (hereafter referred to as the "recipient cell"). They are thought to play a role in transmitting information by transporting components of the secreting cell as contents to the recipient cell. There are two processes by which the contents of exosomes are taken up by recipient cells.
One is endocytosis. In this process, the exosome attaches to the surface of the recipient cell, and the cell membrane envelops the exosome and internalizes it. At this point, the membrane surface markers of the exosome are thought to be involved in the selection and adhesion of the recipient cell. Subsequently, the contents are released from the internalized exosome within the recipient cell.
The other is membrane fusion. Membrane fusion is the process by which the membranes of the exosome and the recipient cell bind to form a single continuous membrane. Proteins and lipids mediating fusion are involved in this process.
The contents of the exosome that enter the recipient cell in this manner act as signals, and the recipient cell responds to those signals.
Accelerating MSC & Exosome
Product & Process Development
Rapid Implementation of Scalable Advanced Therapies
At Aequilibrium Pharma, we are experts in radically simplifying the use of adult human mesenchymal stem/stromal cells (hMSCs) to propel the commercialization of therapeutic technologies. By enabling living cells to become more affordable at the lowest cost per million cells with industry leading quality, we make MSCs easier to access and much simpler to incorporate into product development efforts, leading to rapid acceleration in products coming to market that incorporate these technologies.
Research & Optimization
Our Scalable Bioprocessing Solutions
No matter your scale, our hMSC and exosome systems with built-in processes significantly shorten development timelines and reduce costs on your journey to clinic and market.
Standardized hMSC 3D (Bioreactor) Bioprocess Systems
Scaling hMSC Manufacturing Requires Scaling Each Unit Operation.
Exosomes are “made-to-order” hMSC-derived extracellular vesicles (hMSC-EVs) manufactured in xeno-free conditions using AEQ's optimized extracellular vesicle production platform. You select the quantity of EVs required, tissue source, manufacturing platform, level of downstream processing, and filling concentration, and AEQ will make EVs meeting your specifications. AEQ Exosomes are produced at scales ranging from 1E10 to 1E15 EVs and are analyzed for concentration, purity, and tetraspanin surface markers (CD9, CD63, CD81), with the option to choose additional characterization from our EV analytics portfolio.
Exosomes leverage our advanced equipment capabilities and expertise so you can conduct experiments instead of designing processes to manufacture EVs.
Exosomes are “made-to-order” hMSC-derived extracellular vesicles (hMSC-EVs) manufactured in xeno-free conditions using AEQ's optimized extracellular vesicle production platform. You select the quantity of EVs required, tissue source, manufacturing platform, level of downstream processing, and filling concentration, and AEQ will make EVs meeting your specifications. AEQ Exosomes are produced at scales ranging from 1E10 to 1E15 EVs and are analyzed for concentration, purity, and tetraspanin surface markers (CD9, CD63, CD81), with the option to choose additional characterization from our EV analytics portfolio.
Exosomes leverage our advanced equipment capabilities and expertise so you can conduct experiments instead of designing processes to manufacture EVs.
- Xeno-free human adipose-, bone marrow-, and umbilical cord-derived hMSC-EVs
- Multiple donors available,Autologous 3D organ on chip
- Manufactured using AEQ’s optimized EV production platform
- Produced in 3D microfluidi bioreactor manufacturing systems
- Production of up to 1E15 hMSC-EVs per batch
- Choose from conditioned media, clarified media, concentrated particles (clarification and TFF), and purified EVs (clarification, two rounds of TFF, and chromatography)
- Fill / Finish options: storage buffer, concentration, vial size, vial fill volume
- Analyzed for concentration, purity, and tetraspanin surface markers (CD9, CD63, CD81)
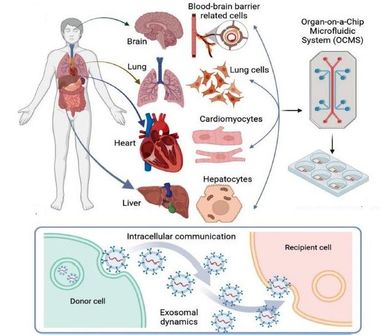
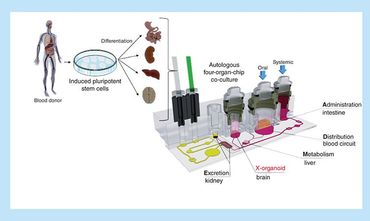
Organ-on-a-Chip System-Autologous Induced Pluripotent Stem Cell-Derived
Organ-on-a-Chip (OOC) technology provides a novel in vitro platform with a possibility of reproducing physiological functions of in vivo tissue, more accurately than conventional cell-based model systems.
The technology opens up great opportunities for next-generation experiments of mimicking human organ functionality, microphysiology and morphology in vitro, replacing traditional animal-based model systems.
By realizing different organ functions on a chip, Organ-on-a-Chip technology is potentially useful for building models of complex diseases. The technology can also be used to study pharmacokentic model when new drugs are being developed.
AEQ customizable Organ-on-Chips system, which is able to recreate the dynamic in-vivo conditions for modeling biochemical and biophysical features of cells' native environment. Combining the PG-MFC flow controller and microfluidic membrane chips, the system provides multi-channel perfusion or reagent recirculation capability to ensure cell nurturing over weeks.



Asset-generation through
our game-changing antibody platform
Transient expression in plants is an effective method for the expression of proteins
Technology Approaches for Bispecific Antibody Development
Leader in plant-derived biologic drug production
Custom Protein GenerationCYP-X production fromgene sequence to crystalgeneration for structural analysisIgM purification to reach 95% purity in SDS-PAGE and HPLC analysisKinase-Y production using insectcell/baculovirus expression systemDevelopability AssessmentFTE-Model Approach
Recombinant antibody production